In-vitro Colorectal Cancer Screening Tests Market Size and Trends 2025-2033
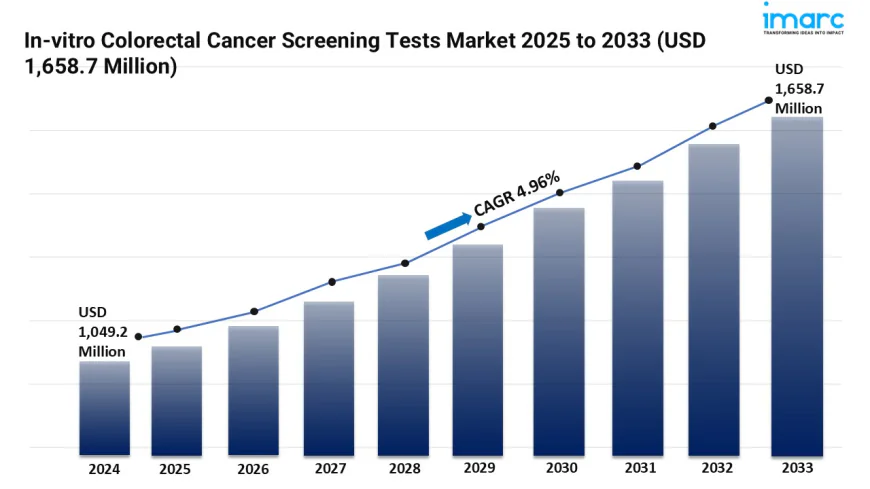
The global in-vitro colorectal cancer screening tests market size was valued at USD 1,049.22 Million in 2024. The market is projected to reach USD 1,658.67 Million by 2033, exhibiting a CAGR of 4.96% from 2025-2033.
Market Overview:
The in-vitro colorectal cancer screening tests market is experiencing rapid growth, driven by Early Detection & Non-Invasive Techniques, Technology Advancements and Sensitivity, and accessibility & decentralized testing. According to IMARC Group's latest research publication, "In-vitro Colorectal Cancer Screening Tests Market : Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global in-vitro colorectal cancer screening tests market size was valued at USD 1,049.22 Million in 2024. The market is projected to reach USD 1,658.67 Million by 2033, exhibiting a CAGR of 4.96% from 2025-2033.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Download a sample PDF of this report: https://www.imarcgroup.com/in-vitro-colorectal-cancer-screening-tests-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends And Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Growth Factors in the In-vitro Colorectal Cancer Screening Tests Industry:
- Early Detection & Non-Invasive Techniques
The global focus on early colorectal cancer detection is driving innovations in non-invasive testing methods. Stool-based DNA and fecal immunochemical tests (FIT) are becoming the preferred first-line tools for population screening due to their comfort, accuracy, and home-use convenience. These tests help identify microscopic traces of blood and genetic mutations long before symptoms appear. Healthcare providers are increasingly promoting such alternatives to improve compliance rates among patients hesitant about colonoscopy. Furthermore, ongoing R&D is exploring RNA markers and microRNA profiling for even earlier detection. The shift toward simple, non-invasive, and scalable tests supports national screening programs aimed at reducing cancer mortality.
- Technology Advancements and Sensitivity
Technological progress is revolutionizing the sensitivity and reliability of in-vitro colorectal cancer screening. Innovations in molecular assays, such as digital PCR, next-generation sequencing (NGS), and microarray platforms, allow detection of rare genetic mutations with exceptional precision. AI-driven image and data analysis tools are minimizing diagnostic errors by interpreting complex biomarker patterns in real time. Companies are also integrating automated sample processing and multiplex testing to reduce turnaround times and human error. The use of liquid biopsy techniques—detecting tumor DNA fragments in blood—is gaining ground as a complementary approach. Collectively, these advancements enhance accuracy, speed, and clinical confidence in early-stage cancer diagnostics.
- Accessibility & Decentralized Testing
Expanding equitable access to colorectal cancer screening remains a core healthcare objective. Self-sampling kits, telehealth consultations, and pharmacy-based test distribution are improving screening participation, particularly in rural and underserved areas. Governments and NGOs are launching national awareness campaigns and subsidy programs to increase adoption rates. Mobile diagnostic vans equipped with stool collection and analysis tools are also bridging healthcare gaps in remote communities. Furthermore, partnerships between diagnostic firms and public hospitals are enabling affordable distribution channels. This decentralized testing approach enhances outreach, empowering individuals to take proactive health measures while easing the burden on centralized healthcare infrastructure.
Key Trends in the In-vitro Colorectal Cancer Screening Tests Market
- Emergence of AI-Powered Diagnostic Algorithms
Artificial intelligence (AI) is transforming colorectal cancer diagnostics by enabling high-accuracy interpretation of complex biological data. Machine learning algorithms can detect subtle genomic and proteomic variations that may indicate early-stage malignancies. AI-powered software is being integrated into laboratory workflows to analyze test results, improving precision and reducing human bias. Predictive analytics platforms are also helping clinicians identify patients at higher risk based on screening patterns and demographic data. These advancements are significantly shortening diagnostic timelines while enhancing clinical decision-making. As AI adoption accelerates, it is expected to become a central component in improving efficiency and predictive accuracy across colorectal cancer screening programs.
- Rising Adoption of Cloud-Based Laboratory Information Systems (LIS)
The implementation of cloud-based laboratory information systems is revolutionizing how diagnostic data is stored, analyzed, and shared. These systems allow seamless collaboration between laboratories, healthcare providers, and public health agencies, ensuring faster communication and better patient management. Cloud integration enhances test traceability, supports real-time quality control, and simplifies large-scale data aggregation for population-level cancer studies. Diagnostic companies are increasingly adopting secure, compliant cloud infrastructures to improve operational scalability. As data-driven healthcare becomes the norm, cloud-based LIS is strengthening the digital backbone of global colorectal cancer screening networks.
- Collaborations for Next-Generation Test Development
Strategic collaborations between biotechnology firms, research institutions, and healthcare organizations are accelerating innovation in in-vitro colorectal cancer screening. Joint ventures and public-private partnerships are fostering the development of multi-omics-based screening solutions that combine genetic, proteomic, and metabolomic insights. Companies are co-developing hybrid diagnostic platforms that integrate molecular biomarkers with AI analytics for greater diagnostic precision. Government grants and research funding are also supporting collaborative efforts to validate new biomarkers and expand screening access in emerging markets. These cross-sector partnerships are laying the groundwork for next-generation colorectal screening technologies with improved accessibility, affordability, and clinical relevance.
Leading Companies Operating in the Global In-vitro Colorectal Cancer Screening Tests Industry:
- Beckman Coulter Inc. (Danaher Corporation)
- Eiken Chemical Co. Ltd
- Epigenomics AG
- Exact Sciences Corporation, Hemosure Inc
- Immunostics Inc. (Boditech Med Inc.)
- Medline Industries LP
- Merck KGaA
- Qiagen N.V
- Quest Diagnostics Incorporated
- R-Biopharm AG
- Siemens AG
- Sysmex Corporation
In-vitro Colorectal Cancer Screening Tests Market Report Segmentation:
Breakup by Product:
- Fecal Occult Blood Tests
- Guaiac FOB Stool Test
- Immuno-FOB Agglutination Test
- Lateral Flow Immuno-FOB Test
- Immuno-FOB ELISA Test
- Biomarker Tests
- Tumor M2-PK Stool Test
- Transferrin Assays
- CRC DNA Screening Tests
- Methylated Gene Testing
- Panel DNA Tests
The in-vitro colorectal cancer screening tests market is segmented by product type into various categories based on diagnostic approaches. Fecal occult blood tests represent a primary segment, which is further divided into guaiac-based FOB stool tests, immuno-FOB agglutination tests, lateral flow immuno-FOB tests, and immuno-FOB ELISA tests.
Breakup by Imaging Type:
- Colonoscopy
- Proctoscopy
- CT Scan
- Ultrasound
- MRI
- PET Scan
By imaging type, the market is categorized into several diagnostic imaging modalities used for colorectal cancer detection. These include colonoscopy and proctoscopy, which are traditional endoscopic procedures used to visually inspect the colon and rectum. Advanced imaging technologies such as CT scans and MRI provide cross-sectional views and detailed anatomical imaging. Ultrasound is employed for non-invasive abdominal evaluations, while PET scans are utilized for detecting cancer spread and monitoring treatment response through metabolic imaging.
Breakup by End User:
- Hospitals
- Clinics
- Diagnostic Laboratories
- Others
In terms of end users, the market serves various healthcare settings. Hospitals represent a major segment where comprehensive diagnostic and treatment services are offered. Clinics, especially specialized gastroenterology centers, play a crucial role in routine screening and follow-up care. Diagnostic laboratories support both public and private sector testing with a wide range of in-vitro diagnostic solutions. The "others" category includes research institutes, academic centers, and home-based test users who increasingly rely on at-home testing kits for preliminary screening.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1–201971–6302










