Saudi Arabia Biosimilar Market Size Landscape and Competitive Outlook 2026-2034
Saudi Arabia biosimilar market size reached USD 664.2 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 1,990.5 Million by 2034, exhibiting a growth rate (CAGR) of 12.97% during 2026-2034.
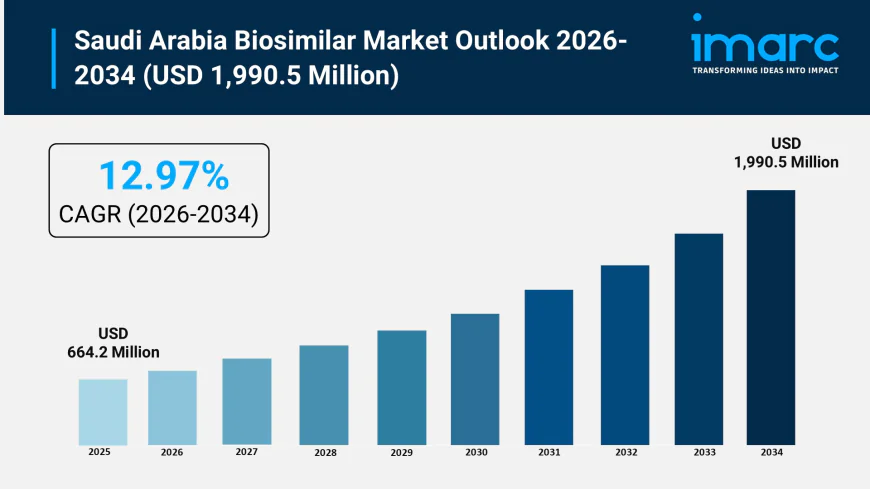
Saudi Arabia Biosimilar Market Overview
Market Size in 2025: USD 664.2 Million
Market Size in 2034: USD 1,990.5 Million
Market Growth Rate 2026-2034: 12.97%
According to IMARC Group's latest research publication, "Saudi Arabia Biosimilar Market: Industry Trends, Share, Size, Growth, Opportunity and Forecast 2026-2034", The Saudi Arabia biosimilar market size was valued at USD 664.2 Million in 2025. Looking forward, IMARC Group estimates the market to reach USD 1,990.5 Million by 2034, exhibiting a CAGR of 12.97% during 2026-2034.
How AI is Reshaping the Future of Saudi Arabia Biosimilar Market
- AI-powered drug discovery platforms accelerate biosimilar development in Saudi Arabia by analyzing molecular structures and predicting immunogenicity profiles, reducing research timelines and enhancing product safety assessments.
- Machine learning algorithms optimize biosimilar manufacturing processes across Saudi production facilities, monitoring critical quality attributes in real-time and ensuring consistent batch-to-batch product uniformity that meets stringent regulatory standards.
- AI-driven pharmacovigilance systems strengthen post-market surveillance of biosimilars in Saudi Arabia, automatically detecting adverse event patterns and supporting regulatory compliance through intelligent data analysis and risk assessment.
- Predictive analytics platforms assist Saudi healthcare providers in biosimilar selection and patient monitoring, analyzing clinical outcomes and cost-effectiveness to optimize treatment decisions across oncology, diabetes, and autoimmune disease management.
- AI chatbots and virtual assistants educate Saudi healthcare professionals and patients about biosimilar efficacy and safety, addressing misconceptions and building confidence in biosimilar adoption through personalized, evidence-based information delivery.
Grab a sample PDF of this report: https://www.imarcgroup.com/saudi-arabia-biosimilar-market/requestsample
How Vision 2030 is Transforming Saudi Arabia Biosimilar Industry
Saudi Arabia’s Vision 2030 is transforming the biosimilar industry by promoting healthcare affordability, pharmaceutical localization, and drug security. Investments in advanced biosimilar manufacturing are reducing import dependence while creating skilled employment and supporting economic diversification. Streamlined regulatory frameworks and international collaboration ensure faster approvals while maintaining global safety and efficacy standards. Biosimilars are improving access to cost-effective treatments for chronic and complex diseases, including oncology and diabetes. Public-private partnerships and biotechnology investments are accelerating innovation and technology transfer. Expanding insurance coverage and modern healthcare infrastructure are driving sustained demand for biosimilars. Preferential procurement policies are supporting rapid market adoption and cost containment. Overall, Vision 2030 positions biosimilars as a cornerstone of Saudi Arabia’s healthcare and pharmaceutical transformation.
Saudi Arabia Biosimilar Market Trends & Drivers:
Saudi Arabia's biosimilar market is experiencing exceptional growth driven by the urgent need to address escalating healthcare costs while maintaining access to advanced biologic therapies for chronic and complex diseases. The Kingdom's substantial healthcare expenditure, combined with rising prevalence of conditions requiring expensive biologic treatments including cancer, rheumatoid arthritis, diabetes, and inflammatory bowel diseases, creates significant demand for cost-effective biosimilar alternatives. Biosimilars offer comparable safety, efficacy, and quality to reference biologics while providing substantial cost savings, making them attractive to both healthcare payers and providers seeking to optimize resource allocation without compromising patient care. The Saudi government's strong commitment to pharmaceutical localization and drug security under Vision 2030 is catalyzing investments in domestic biosimilar manufacturing capabilities, reducing import dependence while building a sustainable local industry.
The regulatory environment in Saudi Arabia has evolved substantially to support biosimilar development and commercialization, with the Saudi Food and Drug Authority implementing comprehensive frameworks that ensure rigorous evaluation while facilitating timely approvals. These regulatory advancements provide clarity and confidence to pharmaceutical companies investing in the Saudi biosimilar market, encouraging both local manufacturers and international partners to expand their portfolios and production capabilities within the Kingdom. Enhanced regulatory harmonization with international standards, including alignment with European Medicines Agency and US Food and Drug Administration guidelines, ensures that Saudi-approved biosimilars meet global quality benchmarks, supporting their acceptance among healthcare professionals and patients.
Saudi Arabia Biosimilar Market Industry Segmentation:
The report has segmented the market into the following categories:
Molecule Insights:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
- Pegfilgrastim
- Trastuzumab
- Bevacizumab
- Others
Indication Insights:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
- Others
Manufacturing Type Insights:
- In-House Manufacturing
- Contract Manufacturing
Breakup by Region:
- Northern and Central Region
- Western Region
- Eastern Region
- Southern Region
Competitive Landscape:
The competitive landscape of the industry has also been examined along with the profiles of the key players.
Recent News and Developments in Saudi Arabia Biosimilar Market
- August 2025: A leading pharmaceutical company inaugurated the first biologics manufacturing facility in the Middle East located in Saudi Arabia, receiving Good Manufacturing Practice approval from the Saudi Food and Drug Authority, establishing a hub for advanced biopharmaceutical production with formulation, fill and finish, and lyophilization capabilities specialized in monoclonal antibodies and complex peptides.
- October 2025: A prominent pharmaceutical organization partnered with an international biosimilar developer to secure commercialization rights for an innovative biosimilar product in Saudi Arabia, leveraging strong local presence and commercial capabilities to expand access to affordable biologic therapies while supporting the Kingdom's pharmaceutical localization objectives.
- December 2025: The Saudi Food and Drug Authority organized a major conference focused on empowering local pharmaceutical manufacturing including biosimilar production, bringing together healthcare providers, regulatory experts, academics, investors, and pharmaceutical companies to strengthen the Kingdom's generic and biosimilar sector through dialogue on regulatory frameworks, investment opportunities, and quality standards.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302










