Plaque Psoriasis Drug Pipeline Analysis: Market Expansion and Future Growth (2034)
Plaque psoriasis is the most prevalent form of psoriasis, affecting an estimated 6.7 million adults worldwide. Characterized by raised, red patches covered with a silvery-white buildup of dead skin cells or scale, plaque psoriasis is a chronic autoimmune skin disease that significantly impacts quality of life.
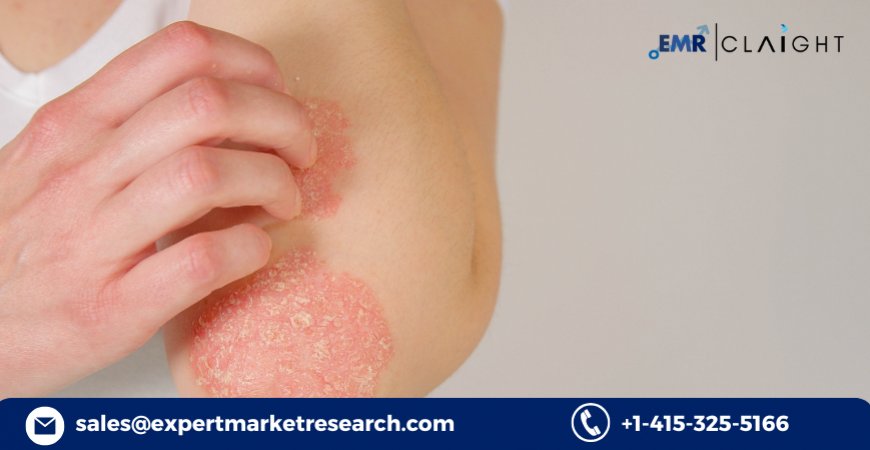
Plaque psoriasis is the most prevalent form of psoriasis, affecting an estimated 6.7 million adults worldwide. Characterized by raised, red patches covered with a silvery-white buildup of dead skin cells or scale, plaque psoriasis is a chronic autoimmune skin disease that significantly impacts quality of life. According to the World Psoriasis Day Consortium, about 2-3% of the global population suffers from some form of psoriasis, and 30% of these patients go on to develop psoriatic arthritis.
The increasing global burden of plaque psoriasis is pushing pharmaceutical companies to invest significantly in drug development and pipeline innovations. With research-driven initiatives aimed at improving efficacy, reducing side effects, and enhancing quality of life, the plaque psoriasis treatment landscape is undergoing transformative change.
Plaque Psoriasis Drug Pipeline Overview
Plaque psoriasis stems from immune system dysfunction that leads to overactive T-cells and excessive skin cell proliferation. Existing treatments include topical therapies, phototherapy, systemic agents, and biologic drugs. However, many of these treatments offer temporary relief, exhibit side effects, or lose effectiveness over time.
Drug development in this space is increasingly focusing on biologics, JAK inhibitors, and immune-targeted therapies that address underlying causes of the disease rather than surface-level symptoms. As a result, a robust pipeline of novel therapies and advanced clinical trials is now being spearheaded by leading pharmaceutical companies globally check out Expert Market Research's Plaque Psoriasis Drug Pipeline Analysis report.
Plaque Psoriasis Market Size and Share
The global plaque psoriasis treatment market was valued at approximately USD 21.3 billion in 2024 and is expected to grow at a CAGR of 6.8% between 2025 and 2030, according to industry projections. The United States, Europe, and Asia-Pacific are major markets, with the U.S. holding the largest share due to higher disease prevalence, increased awareness, and access to advanced biologic therapies.
Biologic drugs, especially IL-17, IL-23, and TNF-alpha inhibitors, currently dominate the market, but next-generation therapies are expected to penetrate rapidly due to ongoing clinical trials and FDA pipeline submissions.
Market Dynamics and Emerging Trends
Rising Disease Prevalence
The steady rise in the number of patients diagnosed with plaque psoriasis, especially in developed countries, is a key factor driving growth.
Shift Towards Biologics and Targeted Therapies
Companies are shifting away from traditional corticosteroids and systemic agents, and moving toward biologics and targeted immune modulators such as interleukin inhibitors (IL-17, IL-23) and JAK inhibitors.
Regulatory Support and Fast-Track Approvals
The FDA and EMA are encouraging innovations through fast-track and orphan drug designations, expediting approvals for novel therapies.
Personalized Medicine and Genomic Insights
Advancements in genetic mapping and precision medicine are allowing tailored treatments for specific patient subgroups, increasing treatment efficacy.
For more information about this report visit
Market Growth Drivers
-
Increased R&D Investment: Pharma companies are investing heavily in research, especially in Phase II and Phase III clinical trials targeting immune pathways.
-
High Unmet Need: There remains a significant demand for safe, effective, and long-term treatment options with fewer side effects.
-
Technological Advancements: Developments in biotechnology, CRISPR gene editing, and AI-based drug discovery are accelerating product innovation.
-
Awareness Campaigns: Initiatives by organizations like the National Psoriasis Foundation (NPF) and World Psoriasis Day are raising disease awareness and treatment options.
Market Opportunities and Challenges
Opportunities
-
Biosimilar Development: With patents expiring on major biologics, there is an increased opportunity for biosimilar entry, offering cost-effective alternatives.
-
Expanding in Emerging Markets: Countries like India, China, and Brazil are seeing an uptick in psoriasis diagnoses, creating untapped growth potential.
-
New Mechanism-of-Action Therapies: Pipeline drugs with novel MOAs offer scope for breakthrough treatments that could dominate niche segments.
Challenges
-
High Treatment Costs: Biologics and targeted therapies remain expensive, limiting accessibility in low-income regions.
-
Side Effects and Long-Term Safety: Long-term impacts of immune-modulating therapies are not yet fully understood.
-
Market Competition: The presence of multiple approved therapies intensifies competition, requiring new drugs to show clear superiority or cost-effectiveness.
Recent Developments in Plaque Psoriasis Drug Pipeline
-
Dermavant Sciences received FDA approval for VTAMA (tapinarof), a steroid-free topical treatment, marking a milestone in non-steroidal therapies.
-
Arcutis Biotherapeutics announced positive results from Phase III trials of roflumilast cream, showing promise as a first-in-class PDE4 inhibitor.
-
Amgen continues its dominance with Enbrel, while also expanding its IL-17 inhibitor trials for broader indications.
-
Novartis is advancing new formulations of Cosentyx (secukinumab), aiming at reducing dosage frequency while maintaining efficacy.
-
Artax Biopharma is innovating T-cell-targeted therapies for autoimmune diseases, including plaque psoriasis, which may revolutionize current standards.
Competitor Analysis: Leading Players in the Pipeline
Power Life Sciences Inc.
A biotechnology company actively involved in autoimmune research. It is currently developing novel immune-regulatory biologics targeting T-cell activation pathways, with applications in plaque psoriasis and related diseases.
Evelo Biosciences, Inc.
Evelo is pioneering oral biologics that target immune-inflammatory diseases through the small intestine. Their drug candidate EDP1815 is in trials for plaque psoriasis and is designed to offer oral, non-systemic relief.
Artax Biopharma Inc
Artax Biopharma focuses on immunomodulation without immune suppression. Their AX-158 drug is being evaluated for T-cell modulation to treat plaque psoriasis with a favorable safety profile.
Bristol-Myers Squibb
A major player with multiple immune-targeted therapies in its pipeline. The company’s acquisition of Celgene and continued expansion into IL-23 targeted drugs has strengthened its dermatology portfolio.
Dermavant Sciences, Inc.
Dermavant’s VTAMA is a game-changer for topical treatments. With continued research into new chemical entities for chronic skin diseases, the company is set to expand further into the psoriasis segment.
Alza Corporation, DE, USA
Part of Johnson & Johnson, Alza Corporation focuses on advanced drug delivery systems. The company plays a key role in patch-based psoriasis therapies and long-acting injectables.
Arcutis Biotherapeutics, Inc.
Known for roflumilast, Arcutis is among the few companies focusing on topical PDE4 inhibitors. They are revolutionizing treatment options for mild to moderate plaque psoriasis.
Shanghai Celgen Bio-Pharmaceutical Co., Ltd
A Chinese biotech working on IL-17 and IL-23 inhibitors targeting autoimmune diseases. They aim to bring affordable biosimilars and biologics to the Asian market.
Amgen
A legacy player with well-established drugs like Enbrel. Amgen continues to invest in next-gen biologics and combination therapies that address resistance or tolerance in long-term use.
Novartis Pharmaceuticals
Novartis remains a market leader with Cosentyx, and continues to innovate through alternative formulations, expanding its reach among treatment-resistant patients.
Huabo Biopharm Co., Ltd.
An emerging player in China developing biosimilars and new biologics for autoimmune conditions, aiming for domestic regulatory approval and future global market expansion.
Galderma R&D
With its dermatology-focused pipeline, Galderma is developing topical and injectable biologics with a focus on patient convenience, rapid onset, and lower cost.
Other Notable Players
These include Pfizer, Eli Lilly, AbbVie, and Johnson & Johnson, all of whom are active in the IL-17 and JAK inhibitor space. Competition among these giants is accelerating innovation and improving patient outcomes.
FAQs on Plaque Psoriasis Drug Pipeline Analysis
What is the most common type of psoriasis?
Plaque psoriasis is the most common type, affecting about 6.7 million adults worldwide.
What are the leading treatments currently available?
Current treatments include biologics (e.g., Enbrel, Cosentyx), topical steroids, systemic immunosuppressants, and new oral therapies like PDE4 inhibitors.
Which drug is considered a game-changer?
VTAMA by Dermavant Sciences is a recent FDA-approved, steroid-free topical drug showing excellent efficacy with minimal side effects.
What are the latest trends in psoriasis drug development?
The focus is on biologic therapies, JAK inhibitors, and novel topical agents with improved safety and efficacy profiles.
What challenges does the drug development landscape face?
High development costs, regulatory hurdles, side effects, and competition among similar drugs pose challenges for new entrants.
How is the competitive landscape evolving?
Both legacy pharmaceutical giants and emerging biotech firms are shaping a highly competitive market with innovative products, leading to enhanced patient outcomes and treatment options.
The plaque psoriasis drug pipeline is experiencing significant momentum due to growing disease prevalence, increased R&D investments, and technological advances. As leading pharma and biotech firms continue to develop next-gen treatments, patients are set to benefit from more effective, safer, and convenient therapies. With multiple late-stage clinical trials and regulatory filings underway, the future of plaque psoriasis treatment appears highly promising, opening up immense market opportunities for both established players and new entrants.
Read More Report:
Gas chromatography market trend
About Us:
Expert Market Research is a leading market research firm delivering data-driven insights to the pharmaceutical, biotechnology, and medical device industries. Our comprehensive research solutions include market research reports, providing in-depth analysis of industry trends and competitive landscapes; drug pipeline reports, tracking drug development progress, clinical trials, and regulatory approvals; epidemiology reports, offering detailed disease prevalence and patient population studies; and patent reports, assessing intellectual property landscapes and innovation trends, among others. Leveraging proprietary data, advanced analytics, and expert methodologies, we help businesses navigate complex markets, optimize strategies, and drive innovation. We empower clients with actionable intelligence, enabling them to make informed decisions and stay ahead in the rapidly evolving healthcare sector.
Media Contact:
Company Name: Claight Corporation
Contact Person: Deepanshu Choudhary, Digital Marketing
Email: [email protected]
Toll Free Number: US +1-415-325-5166 | UK +44-702-402-5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com










