Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Regional Analysis and Forecast 2025–2033
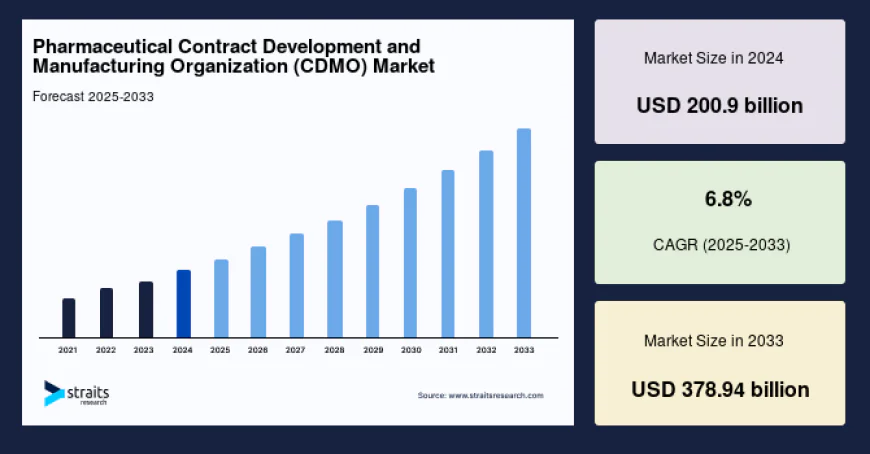
The global pharmaceutical CDMO market size is valued at USD 200.9 billion in 2024 and is projected to reach USD 378.94 billion by 2033
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Outlook:
New York, USA – Straits Research has published a comprehensive new report on the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market, highlighting strong and sustained growth prospects over the forecast period. According to Straits Research, the global pharmaceutical CDMO market size is valued at USD 200.9 billion in 2024 and is projected to reach USD 378.94 billion by 2033, expanding at a compound annual growth rate (CAGR) of 6.8% from 2025 to 2033. The growth reflects the pharmaceutical industry’s increasing reliance on outsourcing to enhance efficiency, reduce costs, and accelerate time-to-market for innovative therapies.
The pharmaceutical CDMO market has become a critical backbone of the global life sciences ecosystem. CDMOs provide end-to-end services ranging from early-stage drug development to commercial-scale manufacturing, packaging, and fill-finish operations. As pharmaceutical pipelines become more complex—particularly with the rise of biologics, cell and gene therapies, and highly potent APIs—drug developers are increasingly turning to specialized CDMO partners with advanced technological capabilities and regulatory expertise.
Market Key Trends
One of the most prominent trends shaping the pharmaceutical CDMO market is the growing demand for biologics and advanced therapies. Biologic drugs, including monoclonal antibodies and recombinant proteins, require sophisticated manufacturing infrastructure and stringent quality controls, driving pharmaceutical companies to collaborate with experienced CDMOs. Additionally, the integration of development and manufacturing services under a single provider is gaining traction, enabling seamless scale-up from clinical to commercial production.
Another key trend is the adoption of digitalization and automation across CDMO operations. Technologies such as continuous manufacturing, advanced analytics, and digital quality management systems are improving productivity, compliance, and batch consistency. Furthermore, capacity expansion and strategic partnerships among leading CDMOs are reshaping the competitive landscape, as companies seek to strengthen their global footprint and service portfolios.
Request Sample @ https://straitsresearch.com/report/pharmaceutical-contract-development-and-manufacturing-organization-market/request-sample
Driving Factors
The primary driver of the pharmaceutical CDMO market is the increasing outsourcing of pharmaceutical R&D and manufacturing activities. Pharmaceutical companies, both large and small, are under pressure to optimize operational costs while maintaining high quality and regulatory compliance. Outsourcing allows them to focus on core competencies such as drug discovery, marketing, and commercialization.
Another major growth driver is the surge in clinical trial activity worldwide, fueled by rising prevalence of chronic diseases, oncology indications, and rare disorders. As clinical pipelines expand, demand for CDMO services across pre-clinical and clinical phases continues to rise. In addition, stringent regulatory requirements across major markets encourage companies to rely on CDMOs with proven compliance track records and global regulatory experience.
Opportunities
The pharmaceutical CDMO market presents significant opportunities in emerging economies, where increasing pharmaceutical production, favorable government initiatives, and lower manufacturing costs are attracting global sponsors. Moreover, the rapid growth of small and mid-sized pharmaceutical and biotech companies offers lucrative opportunities for CDMOs, as these firms often lack in-house manufacturing capabilities.
Another promising opportunity lies in high-potency APIs, biologics, and personalized medicines, which require specialized containment, equipment, and technical expertise. CDMOs that invest in advanced manufacturing platforms and flexible facilities are well positioned to capitalize on these high-growth segments.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Segmentation
- By Service Type
- Drug Development Services
- Pharmaceutical Manufacturing Services
- Pharmaceutical API Manufacturing Services
- Pharmaceutical FDF Manufacturing Services
- Biologics Manufacturing Services
- Biologics API Manufacturing Services
- Biologics FDF Manufacturing Services
- Packaging & Labelling Services
- Fill-finish Services
- Others
- By Research Phase
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- By End-User
- Big Pharmaceutical Companies
- Small & Mid-Sized Pharmaceutical Companies
- Generic Pharmaceutical Companies
- Others
Get Detailed Segmentation @
https://straitsresearch.com/report/pharmaceutical-contract-development-and-manufacturing-organization-market/segmentation
List of Key Players in Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
- Thermo Fisher Scientific, Inc.
- Lonza Group
- WuXi Apptec
- WuXi Biologics
- AbbVie, Inc.
- Catalent, Inc.
- Samsung Biologics
- Evonik Industries AG
- FUJIFILM Holding Corporation
- Siegfried Holding AG
- Boehringer Ingelheim International
- Merck KGaA
- Almac Group
- Charles River Laboratories
- Asychem Inc.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Geographic Analysis
Geographically, North America dominates the pharmaceutical CDMO market due to its strong pharmaceutical and biotechnology base, high R&D spending, and presence of leading CDMO players. Europe follows closely, supported by a robust regulatory framework and increasing biologics manufacturing activities. Meanwhile, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, driven by expanding manufacturing capacity, cost advantages, and rising investments in pharmaceutical R&D across countries such as China and India.
Conclusion
The global pharmaceutical CDMO market is set for sustained growth as outsourcing becomes an integral strategy for pharmaceutical companies navigating complex drug development and manufacturing landscapes. With rising demand for biologics, advanced therapies, and flexible manufacturing solutions, CDMOs will continue to play a pivotal role in accelerating innovation and ensuring supply chain resilience. Companies that invest in advanced technologies, regulatory compliance, and global capacity expansion are expected to gain a competitive edge in this evolving market.
Have Any Query? Ask Our Experts @
https://straitsresearch.com/buy-now/pharmaceutical-contract-development-and-manufacturing-organization-market
Why Straits Research?
Straits Research is a leading market intelligence firm providing actionable insights, in-depth analysis, and data-driven forecasts across diverse industries. With a strong focus on accuracy and strategic relevance, Straits Research empowers organizations to make informed business decisions and stay ahead in competitive global markets.










